非线性特性也和其他生物体中的生命特性一样易受到很多内外界因素的调节和制约,具有易损性。这是作为一个生命体系中才有的特征,现已明确手术创伤和动物死亡都会影响基底膜反应的灵敏度。动物死亡后,基底膜对BF附近低强度声刺激的反应灵敏度下降,调谐变差。因此导致基底膜反应的压缩式非线性现象在数分钟后消失。各种原因引起的耳蜗功能损害可导致基底膜振动相位的变化,如相位的滞后和BF附近带延迟的减小。两音相互调制畸变产物(Distortion production otoacoustic emissions,DPOAE),如2f1-f2的第3个频率(f3)的幅度,也会因为耳蜗功能的损害而减弱或消失。
(一)噪声暴露
强度过高的噪声刺激对基底膜反应的影响与动物死亡的影响相似:基底膜反应变成线性,BF处灵敏度下降,调谐变差,反应峰值向低频方向移动大约半个倍频程。对胞内电位的研究也发现强噪声暴露后,毛细胞感受器电位的输入/输出曲线的非线性趋向消失,而线性会增强(见图14-2B)。在中阶记录的场电位CM的输入/输出曲线也表现为非线性减弱(图14-7)。两音抑制现象减弱(图14-8)。

图14-7 白噪声暴露前后中阶记录的CM(0.8kHz)的I/O曲线(n=8)
随暴露次数增加曲线非线性特点减弱,CM幅度减小
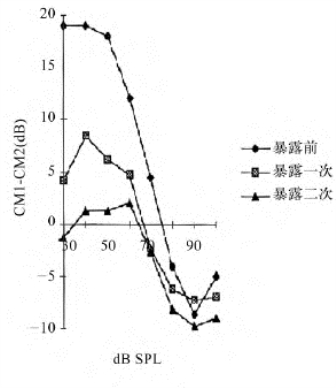
图14-8 CM抑制幅度在暴露前后的变化
图14-8中f1为探测音0.8kHz(CM1),f2为抑制音0.7kHz(CM2),CM1-CM2为两音抑制效应,图左侧30~70dB CM1-CM2>0提示加抑制音可使CM幅度降低;图右侧70~100dB CM1-CM2<0表示加抑制音后反使CM幅度增大,三条曲线的变化趋势可见随暴露噪声次数的增加,CM1-CM2幅度减少即抑制音产生的抑制效果降低。
噪声暴露可以使豚鼠耳蜗外淋巴液中谷氨酸含量增加,还可以使内毛细胞释放过多的谷氨酸产生兴奋性毒性,直接引起传入传出突触复合体的损伤,CAP的输入-输出曲线的非线性消失。
噪声也会影响静纤毛的结构,而静纤毛在耳蜗放大器的工作中是起作用的,噪声的刺激会引起外毛细胞纤毛上机械-电换能通道的功能失调,影响毛细胞感受器电位的产生,进而影响耳蜗放大机制,因此噪声的暴露会使得耳蜗的主动机制遭到一定程度的破坏(OHC损伤),这种由于耳蜗基底膜的微结构产生的破坏比突触功能的失调更容易出现在噪声引起的损伤中,同样会破坏耳蜗的主动机制,导致了暂时性阈移(图14-9)。

图14-9 豚鼠在100dB白噪声暴露2h后,圆窗龛记录的CM的I/O曲线示非线性特点消失,形态学证明以OHC损伤为主
(二)药物影响
一些影响耳蜗功能的药物,如呋塞米、水杨酸、奎宁等,能直接影响到基底膜的正反馈机制从而影响到耳蜗非线性特性的表达。
1.呋塞米 是一种“襻抑制”利尿药,它能够可逆性地消除蜗内电位(EP),降低机-电能转换,使内毛细胞和外毛细胞感受器电位下降,呋塞米能使BF处基底膜振动幅度降低大约60dB,外毛细胞感受器电位很小的变化就能够导致基底膜机械振动的大幅度变化,从而大幅度地改变耳蜗功能。呋塞米使得基底膜的灵敏度下降且呈线性,这种变化是BF特异性的,也是可逆的。提示外毛细胞与基底膜之间的联结构成了一个高增益的反馈系统。
2.奎宁 是一种传统的抗疟疾药,它能够可逆性地引起听力下降和耳鸣。奎宁耳毒性的特点之一是直接影响外毛细胞的电致运动,而对EP无影响。将奎宁静脉注射或直接灌注到耳蜗鼓阶,能使基底膜的振动变为线性,频率调谐变差,BF向低频侧移动,灵敏度下降达15dB以上。
3.水杨酸 也可以可逆性地引起听力下降和耳鸣。向外淋巴液灌注2mmol/L水杨酸能够明显降低基底膜反应的灵敏度,使基底膜调谐变差,BF向低频侧移动。水杨酸在离体实验中已被证明能够降低外毛细胞的电能动性和轴向劲度。因此,奎宁和水杨酸对基底膜振动的影响,说明了外毛细胞独特的机械特性在耳蜗放大功能中的重要作用。
(三)其他内耳疾病
梅尼埃病患者的耳蜗电图CAP的I/O线性化,原来正常时的“L”段和“平台”段消失,只剩“H”段(图9-18)。呈现耳蜗病变的响度重振现象。
总之,随着耳蜗运动的非线性特性的发现,也推动了耳蜗机械学的研究和探索,也产生了一个研究耳蜗机械学的数学模型的分支学科,这对耳蜗非线性的研究起到了很大的促进作用。而反之要具有非线性振动特性的要求本身也成了测量一个耳蜗模型是否成功的主要依据。
耳蜗非线性特性的丧失可以使得基底膜调谐曲线变宽,导致了响度重振、频率选择性变差、听力减退等表现,这些后果反映在听力症状上也会影响对言语的分辨能力。因此对言语信号的编码等处理有可能与耳蜗非线性特性有关。那么我们对耳蜗非线性特性通过各种手段测量后进行准确评估,是否可以将由于耳蜗功能减退而引起的言语识别功能下降与听觉中枢疾病引起的言语识别功能下降综合征鉴别开来呢?
在已经阐明了外毛细胞的电致运动在耳蜗非线性中的耳蜗放大功能的具体机制后,外毛细胞的这种放大作用是如何实现提高内毛细胞的频率选择性的;外毛细胞的机械放大作用是如何耦合到内毛细胞的;及外毛细胞的放大功能是如何实现自动增益控制的等都是复杂但有趣的问题,也都是值得深入探讨的问题。总之,耳蜗非线性特性的深入研究,必将大大促进助听听力学的发展。
(张 倩 李兴启)
1 Bekesy G,Experiments in Hearing,New York:John Wiley and Sons,1960
2 Benser ME,Marquis RE,Hudspeth AJ.Rapid,active hair bundle movements in hair cells from the bullfrog’s sacculus.J Neurosci,1996,16:5629
3 Brown AM,Gaskill S A,Williams DM,Mechanical filtering of sound in the inner ear.Proc R Soc Lond B Biol Sci,1992,250(1327):29
4 Brown MC,de Venecia RK,Guinan JJ Jr.Responses of medial olivocochlear neurons,specifying the central pathways of the medial olivocochlear reflex.Exp Brain Res,2003,153(4):491
5 Brownell WE.Evoked mechanical responses of i-solated cochlear outer hair cells.Science,1985,227:194
6 Cantos R,Lopez DE,Merchan JA,et al.Olivocochlear efferent innervation of the organ of corti in hypothyroid rats.J Comp Neurol,2003,459 (4):454
7 Chan DK,Hudspeth AJ.Ca2+current-driven nonlinear amplification by the mammalian cochlea in vitro.Nat Neurosci,2005,8:149
8 Cheatham MA,Dallos P.Comparison of low and high side two-tone suppression in inner hair cell and organ of Corti responses.Hear Res,1990,50:193
9 Cooper NP,Rhode WS.Basilar membrane mechanics in the hook region of cat and guinea-pig cochleae:sharp tuning and nonlinearity in the absence of baseline position shifts.Hear Res,1992,63(1-2):163
10 Cooper NP,Rhode WS.Mechanical responses to two-tone distortion products in the apical and basal turns of the mammalian cochlea.J Neurophysiol,1997,78(1):261
11 Crawford AC,Fettiplace R.The mechanical properties of ciliary bundles of turtle cochlear hair cells.J Physiol(Lond),1985,364:359
12 De Boer E.Mechanics of the cochlea:modeling efforts,in The Cochlea,Dallos P,Poper AN,Fay RR.Editors,Springer-Verlag:New York,1996:258-317
13 Davis H.An activeprocess in cochlear mechanics.Hearing Research,1983,9:79
14 Dallos.P,Schoeny ZG,Cheatham MA.Cochlear summating potentials.Descriptive aspects.Acta ctolaryngol suppl,1972,302:1
15 Dallos P.The auditory perphery:biophysics and physiology,New York:Academic Press,1973
16 Dallos PR.Response characteristics of mammalian cochlear hair cells.J Neurosci,1985,5:1591
17 Dallos P,Evans BN,Hallworth R.Nature of the motor element in electrokinetics shape changes of cochlear outer hair cells.Nature,1991,350 (6314):155
18 Dallos P.,Evans B.N.High-frequency motility of outer hair cells and the cochlear amplifier.Science,1995,267(5206):2006
19 Frank G,Hemmert W,Gummer AW.Limiting dynamics of high-frequency electromechanical transduction of outer hair cells.Proc Natl Acad Sci U S A,1999,96(8):4420
20 Gold T,HearingⅡ.The physical basis of the action of the cochlea.Proc R Soc Lond B Biol Sci,1948,135:492
21 Groff JA,Liberman MC.Modulation of cochlear afferent response by the lateral olivocochlear system:activation via electrical stimulation of the inferior colliculus.J Neurophysiol,2003,90:3178
22 He DZ,Evans BN,Dallos P.First appearance and development of electromotility in neonatal gerbil outer hair cells.Hear Res,1994,78(1):77 23He DZ.Chick hair cells do not exhibit voltagedependent somatic motility.J Physiol,2003,546 (Pt 2):511
24 Hodgkin AL,Huxley AF.A quantitative description of membrane current and its application to conduction and excitation in nerve,1952.Review)Bull Math Biol,1990,52(1-2):25
25 Hudspeth A,Mechanical amplification of stimuli by hair cells.Curr Opin Neurobiol,1997,7(4):480
26 Hubbar dAE,Mountain DC.Alternating current delivered into the scala media alters sound pressure at the ear drum.Science,1983,222:510
27 Kempt DT.Evidence of mechanical nonlinearity and frequency selective wave amplification in the cochlea.Arch Otorhinolaryngol,1979,224:37
28 Kolston PJ.What type of force does the cochlear amplifier produce?J Acoust Soc Am,1990,88 (4):1794
29 Kros C.Aid from hair force.Nature,2005,433:810
30 LePage E.L,Johnstone B.M,Nonlinear mechanical behavior of the basilar membrane in the basal turn of the guinea pig cochlea.Hear Res,1980,2(3-4):183
31 Liberman MC.Effects of chronic cochlear de-efferentation on auditory-nerve response.Hear Res,1990,49:209
32 Martin P,Hudspeth AJ.Active hair-bundle movements can amplify a hair cell’s response to oscillatory mechanical stimuli.Proc Natl Acad Sci U S A,1999,96:14306
33 Mountain DC.Changes in endolymphatic potential and crossed olivocochlear bundle stimulation alters cochlear mechanics,Science,1980,210:71
34 Murugasu E,Russell IJ.Salicylate ototoxicity:the effects on basilar membrane displacement,cochlear microphonics,and neural responses in the basal turn of the guinea pig cochlea.Aud Neurosci,1995,1:139
35 Narayan SS.Frequency tuning of basilar mem-brane and auditory nerve fibers in the same cochleae.Science,1998,282(5395):1882
36 Neely ST,Kim DO.An active cochlear model showing sharp tuning and high sensitivity.Hear Res,1983,9(2):123
37 Neely ST,Kim DO.A model of active elements in cochlear biomechanics.J Acoust Soc Am,1986,79:1472
38 Niu X,Canlon B.The signal transduction pathway for the dopamine D1receptor in the guineapig cochlea.Neuroscience,2006,137:981
39 Nuttall AL,Dolan DF,Avinash G..Measurements of basilar membrane tuning and distortion with laser velocimetry,in The Mechanics and Biophysics of Hearing,P.Dallos,et al.,Editors,Springer-Verlag:Berlin,1990:295-299
40 Nuttall AL.Basilar membrane motion and position changes induced by direct current stimulation,In Active Hearing,A.Flock,D.Ottoson,and M.Ulfendahl,Editors,Elsevier Science Ltd:Oxford,1995:283-293
41 Nuttall AL,Dolan DF.Steady-state sinusoidal velocity responses of the basilar membrane in guinea.J Acoust Soc Am,1996,99(3):1556
42 Nuttall AL,Dolan DF.Two-tone suppression of inner hair cell and basilar membrane responses in.J Acoust Soc Am,1993,93(1):390
43 Oliver D.Intracellular anions as the voltage sensor of prestin,the outer hair cell motor protein.Science,2001,292(5525):2340
44 Palmada M,Centelles JJ.Excitatory amino acid neurotransmission pathway for metabolism,storage and reuptake of glutamate in brain.Front Biosci,1998,20(3):701
45 Patuzzi R.Sellick PM.A comparison between basilar membrane and inner hair cell receptor potential input-output functions in the guinea pig cochlea.J Acoust Soc Am,1983,74(6):1734
46 Pickles JO.An introduction to the physiology of hearing,1988,London,San Diego:Academic Press
47 Puel JL.Chemical synaptic transmission in the cochlea.Prog Neurobiol,1995,47:449
48 Rajan R.Centrifugal pathways protect hearing sensitivity at the cochlea in noisy environments that exacerbate the damage induced by loud sound.J.Neurosci,2000,20(17):6684
49 Rhode L,Ruggero MA,Rich NC.Two-tone distortion on the basilar membrane of the chinchilla cochlea.J Neurophysiol,1997,77(5):2385
50 Rhode WS.Observations of vibration of the basilar membrane in squirrel monkeys using the Mossbauer technique.J Acoust Soc Am,1971,49 (Supp 2):1218
51 Rhode WS,Robles L.Evidence from Mossbauer experiments for nonlinear vibration in the cochlea.J Acoust Soc Am,1974,55:588
52 Rhode WS,Cooper NP.Two-tone suppression and distortion production on the basilar membrane in the hook region of cat and guinea pig cochlea.Hear Res,1993,66:31
53 Rhode WS.An investigation of postmortem cochlear mechanics using the Mossbauer effect,in Basic Mechanisms in Hearing,A.R.Moller,Editor,Academic:New York,1973:49-63
54 Rhode WS.Some observations on two-tone interaction measured with the Mossbauer effect,in Psychophysics and Physiology of Hearing,E.F.Evans and J.P.Wilson,Editors,Academic Press,London,1977:27-41
55 Robles L,Ruggero MA,Rich NC.Two-tone distortion in the basilar membrane of the cochlea.Nature,1991,349(6308):413
56 Robles L,Ruggero MA.Mechanics of the mammalian cochlea.Physiol Rev,2001,81(3):1305
57 Rhode WS,Cochlear prrtition vibration-recent views.J Acoust Soc Am,1980,67(5):1696
58 Ruel J,Nouvian R,d’Aldin C,et al.Dopamine inhibition of auditory nerve activity in the adult mammalian cochlea.Eur J Neurosci,2001,14:977
59 Rucci A,Fettiplace R.The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells.J Physiol(Lond),1997,501(Pt 1):111
60 Rucci A,Crawford AC,Fettiplace R.Mechanisms of active hair bundle motion in auditory hair cells.J Neurosci,2002,22(1):44
61 Rucci A.Active hair bundle movements and the cochlear amplifier.J Am Acad Audiol,2003,14 (6):325
62 Ruggero M.The effects of acoustic trauma,other cochlear injury,and death on basilarmembrane responses to sound,in Scientific Basis of Noise-Induced Hearing Loss,A.Axelsson,et al.,Editors,Thieme Medical Publishers:New York,1996:23-35
63 Ruggero MA,Robles L,Rich NC.Two-tone suppression in the basilar membrane of the cochlea:mechanical basis of auditory-nerve rate suppression.J.Neurophysiol,1992,68(4):1087
64 Ruggero M.The effects of acoustic trauma,other cochlear injury,and death on basilar membrane responses to sound,in Scientific Basis of Noise-Induced Hearing Loss,A.Axelsson,et al,Editors,Thieme Medical Publishers:New York,1996:23-35
65 Sachs MB,Abbas PJ.Rate versus level functions for auditory-nerve fibers in cats:Tone-burst stimuli.J Acoust Soc Am,1974,56:1835
66 Sewell WF.The effects of furosemide on the endocochlear potential and auditory nerve fiber tuning curves in cats.Hear Res,1984,14:305
67 Sun W,Salvi RJ.Dopamine modulates sodium currents in cochlear spiral ganglion neurons.Neuroreport,2001,12:803
68 Tasaki I,Davis H,Legouix JP.The space-time pattern of the cochlear microphonics(guinea pig),as recorded by differential electrodes.J Acoust Soc Am,1952,24:502
69 Warren EH 3rd,Liberman MC.Effects of contralateral sound on auditory-nerve responses.I.Contributions of cochlear efferents.Hear Res,1989,37(2):89
70 Xingqi Li,Ning Yu,Jianhe Sun.Prophylactic effect of Ca2+-deficient artificial perilymph perfusion on noise-induced hearing loss.Chinese Medical Journal,2003,3:156
71 Zheng J.Alterations of basilar membrane veloci-ty responses by scala tympani perfusion of quinine in guinea pig cochlea.Abstr Assoc Res Otolaryngol,2002,25:533
72 Zheng XY,Henderson D,McFadden SL,et al.Auditory nerve fiber responses following chronic cochlear de-efferentation.J Comp Neurol,1999,406:72
73 Zenner HP,Zimmermann U,Schmitt U.Reversible contraction of isolated mammalian cochlear hair cells.Hear Res,1985,18(2):127
75 侯志强,余力生,李兴启.外侧橄榄耳蜗束在内毛细胞下突触复合体中的调控作用.听力学及言语疾病杂志,2007,5:113
76 李士勇.非线性科学与复杂性科学.哈尔滨:哈尔滨工业大学出版社,2006
77 李兴启,姜泗长.耳蜗电图在临床上的诊断价值及耳蜗毛细胞生理功能的观察.军医进修学院,1982,3(1):20
78 李兴启,孙 伟.不同频率短纯音(tone burst)在中阶记录SP的特点.听力学及言语疾病杂志,1996,4(4):172
79 李兴启,申卫东,卢云云,等.从内毛细胞下突触复合体结构和功能看听神经病的发病机制及部位——读书心得.听力学及言语疾病杂志,2005,13(4):223
80 李兴启.几种常用听觉诱发反应测听的比较.中国听力语言康复科学杂志,2008,(4):10
81 马尔萨斯.人口原理.兰州:敦煌文艺出版社,2007
82 孙 勍,李兴启,单希征,等.噪声对豚鼠耳蜗电位及其超微结构的影响.中国耳鼻咽喉-头颈外科杂志,2005(6):15
83 孙 伟,李兴启,姜泗长.白噪声暴露后豚鼠耳蜗两音抑制现象的实验观察.声学学报,1997,22(4):299
84 孙 伟,李兴启,姜泗长.白噪声暴露对豚鼠耳蜗毛细胞感受的电位非线性特性的影响.中华耳鼻咽喉科杂志,1997,32(2):88
85 王 坚,蒋 涛,曾凡刚,等.听觉科学概论.北京:中国科学技术出版社,2005:144-146
免责声明:以上内容源自网络,版权归原作者所有,如有侵犯您的原创版权请告知,我们将尽快删除相关内容。