(一)听觉中枢的可塑性
许多现象都表明,听觉中枢可能存在可塑性(plasticity),即频率重组(reorganization)。过去双侧听觉障碍患者常规单耳配戴助听器,经过一段时间后,未配戴耳往往比配戴耳的言语识别率低,这与国人观察到的单侧耳聋患者配戴助听器后,对侧耳(健耳)DPOAE幅度升高的现象似乎有些不谋而合。前者结果提示双侧耳聋必须双侧配戴助听器;而后者提示,单耳聋也须配戴助听器。但在另一些试验中,一侧耳长时间配戴助听器后,配戴耳在高声强(>75dB SPL)时,在噪声环境中的言语识别率要好于未配戴耳,这可能是配戴耳对强度敏感性增加的结果。这些现象似乎都证明了听觉中枢存在可塑性。
在研究不同感觉皮质可塑性时,发现视力丧失者的听觉较常人敏感,而听功能丧失者的视觉较常人敏感。且有调查表明后天性(十几岁以后)失明者的听力并不像前述那样敏感,只有先天性视力丧失者听觉较常人敏感。更支持这种假说的是:人们利用功能磁共振成像检查发现,音乐指挥家的听皮质对钢琴音的反应区域显著大于一般人,且开始学习音乐的年龄越小,此听皮质反应区域越大。这些现象均提示声学环境以及学习获得与中枢可塑性密切相关。
真正对中枢可塑性的研究,是始于听觉中枢神经元特征频率(characteristic frequency,CF)建立和在皮质音频排列定位以后(图2-19)。后者的变化可作为中枢可塑性的最有效和最可靠的检测指标。不少研究表明,耳蜗的形态和功能改变,会导致听觉中枢系统结构和功能的变化,其典型变化就是听觉中枢音频定位图的重组,因此有作者又将可塑性称之为频率重组。
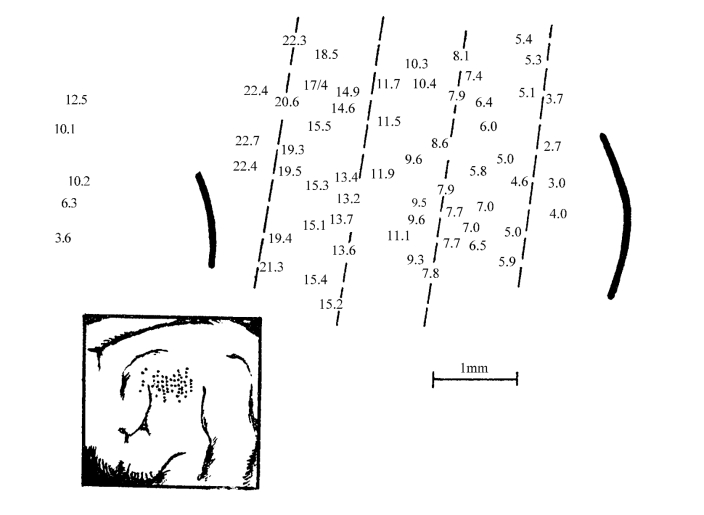
图2-19 听皮质表面的声音频率投射图(猫)
数字表示该区细胞反应的最优频率(kHz);左下角照片示记录电极所在位置(自梁之安)
当选择性破坏耳蜗基膜某一频段(如2kHz)毛细胞后,相对应的听皮质2kHz特征性频率敏感区暂时受到抑制(即反应阈提高),但相隔相当长的时间后,2kHz处以外的其他CF区逐渐对2kHz声音有了反应,这可能是听觉中枢音频定位图的重组。但又有实验表明,鼬鼠耳蜗急性切除后24h产生未受损耳蜗侧的皮质。神经元平均阈值降低和自发放电率升高,说明听觉系统可兴奋性变化在很短的时间内出现。但耳蜗切除后,随存活时间的延长,同侧耳兴奋的部位越来越多。神经元反应阈值也越来越低。耳蜗切除的动物可作为先天性耳聋的模型。上述试验结果提示,电子耳蜗置入年龄的选择和蜗性聋的持续时间,对电子耳蜗置入效果至关重要。用PET观察听皮质糖代谢区,发现年龄小的患儿颞叶皮质中存在较宽的摄取降低区,随着年龄的增长,上述区域逐渐缩小,提示先天性蜗性聋患儿电子耳蜗置入越早越好。可以用PET检测颞叶低糖代谢区的大小作为选择电子耳蜗植入候选者及置入后效果评估的指标。如果在置入人工耳蜗前,从皮质显示的新陈代谢处于正常水平的话,表明由于神经系统的重组,听觉区域已由非听觉突触占据,人工耳蜗置入后效果可能不佳。用耳毒性抗生素诱发高频听力损失后,存活12个月的成年猫听区皮质发生了广泛重组。在正常非损伤时猫的高频可兴奋的区域被相邻低频区所代替。
对于高频听力丧失的重度感音神经性聋患者,配戴数字移频助听器并经过语训可明显提高语言理解能力。相对于传统助听器,数字移频助听器将患者无法听到的高频区声信号按比例压缩到低频区域,并保持声音的波形特点。初配助听器的患者对移频后的声音并不能掌握,但经过一段时间的语训后,患者对语言的理解能力显著提高,在此过程中,听觉中枢的重组可能发挥了重要的作用。
(二)听觉中枢可塑性神经机制
1.侧抑制效应 在正常情况下,耳蜗基膜上不同频率部位的传入冲动及听觉中枢各级平面的不同频率感受区域都存在内在的抑制作用,即侧抑制。
(1)感受细胞间的侧抑制(当某一感受细胞兴奋时,同时对相邻的细胞产生抑制):当基膜某个最佳频率部位被破坏后,该区域的感觉细胞的电生理活动发生障碍,相应的传导束无兴奋性活动传入,但同时减弱了对邻近部位的传入冲动的抑制,其相应的听觉中枢的音频感受区的细胞活动性增强,取代损害部位的细胞而执行功能。
(2)突触侧抑制效应(synapse effectiveness),被损害区域的神经元与邻近区域的神经元间的被抑制的突触活性增强;在两个邻近区域之间形成新的突触。
(3)邻近区域的听觉神经元的树突与来自丘脑皮质终支形成的突触的活性增强。
2.下行通路调控
(1)近年来一些研究认为在听觉中枢信号传递通路中,最为有效的抑制性神经递质之一是γ-氨基丁酸(GABA)。在下丘(inferior colliculus,IC)已观察到GABA阳性的神经元,耳蜗损伤后螺旋神经元释放的GABA减少,因而造成GABA抑制性作用的下降可能是造成IC活动过强的原因。Milbrandt等应用免疫组织化学方法检测了耳蜗神经元内谷氨酸脱羧酶(GAD,GABA复合物中的一种酶)的水平,结果显示噪声性耳蜗损伤后,GAD急剧下降。另外,Salvi等利用荷包牡丹碱可与GABA结合的特性,将其注入正常动物的大脑听皮质(acoustic cortex,AC),以期阻断GABA介导的抑制,结果显示AC活动亢进;而当荷包牡丹碱注入IHC受损的灰鼠大脑AC,它对AC的局域电位几乎没有影响,证实GABA抑制性作用的消失可能是继IHC破坏后大脑皮质反应增强的原因。
(2)有作者研究了经典条件反射对大棕蝠下丘神经元CF的影响。结果表明训练后下丘神经元的CF移向条件刺激纯音的频率。这一变化与皮质刺激引起的变化相一致。当用GABA的竞争性拮抗剂阻断听觉皮质及皮质下行系统后再行条件反射训练,则观察不到上述CF漂移现象,而若在动物形成条件反射后再阻断听觉皮质及皮质下行系统的电活动,则不影响上述可塑性变化的产生与维持。这些实验更直观地证明了皮质下行调控在学习诱导的中枢听觉系统可塑性变化中起着重要的作用。
3.分子生物学机制 有研究表明,在大鼠耳蜗内使用电刺激时,听觉系统接受的是一种不被识别的单音调的刺激,但2h后,神经元通过改变基因表达模式而对这个全新的听觉经验发生反应,称即时基因反应。
依赖于耳蜗受刺激的部位,腹侧耳蜗核的腹侧或背侧部分神经元以耳蜗音位方式(cochleotopic manner)出现反应。这同样可见于橄榄复合体和下丘。可以推测,即时基因反应的早期产物作为一种转录因素(transcription factors),之后进一步激发了大量的基因表达。同时存在细胞内信号的大量交换,包括中枢神经元网络的再构建。其中一个基因c-fos的下游产物是GAP-43,后者出现于听力损伤数天或数周后,而非数小时后,这显示了后续突触水平的可塑性调整。
(郑杰夫 李兴启)
1 Bekesy G.Experiments in Hearing.New York:John Wiley &Sons,1960
2 Brownell EW.Outer hair cell electromobility and otoacoustic emissions.Ear Hear,1990,11:82
3 Bartlett EL,Stark JM,Gullery RW,et al.Comparison of the fine structure of cortical and collicular terminals in the rat medial geniculate body.Neuroscience,2000,100:811
4 Bourassa J,Pinaulat D,Deschenes M.Corticothalamic projections from the cortical barrel field to the somatosensory thalamus in rats:A siglegiber study using biocytin as an anterograde tracer.Ear J Neurosci,1995,7:19
5 Brugge JF,Anderson DJ,Hind JE,et al.Time structure of discharges in single auditory nerve fibers of the squirrel monkey in response to complex periodic sounds.J Neurophysiol,1969,32(3):386
6 Budinger E,Heil P,Scheich H.Functional ofganization of auditory cortex in the Morgolian gerbil(Meriones unguiculatus).Ⅳ.Connections with anatomically characterized subcortical structures.Eur J Neurosci,2000,12(7):2452
7 Cetas JS,Price RO,Velenovsky DS,et al.Cell types and response properties of neurons in the ventral division of the medial geniculate body of the rabbit.J Comp Neural,2002,445(1):78
8 Contreras D,Destexhe A,Sejnowski TJ,et al.Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback.Science,1996,274:771
9 Dallos,P.,The active cochlea.J Neurosci,1992.12(12):4575
10 Dallos P,Popper AN,and Fay RR(Eds).The Cochlea,1996,Springer-Verlag:New York.
11 Darian-Smith C,Tan A,Edwards S.Conparing thalamocortical and corticothalamic microstructure and spatial reciprocity in the macaque ventral posterolateral nucleus(VPLc)and medial pulvinar.J Comp Neurol,1999,410:211
12 Gulick WL,Gescheider GA,Frisina RD(Eds).Hearing Physiological Acoustics,Neural Coking,and Psychoacoustics,Oxford University Press:New York,1989
13 Golshani P,Liu XB,Jones EG.Differences in quantal amplitude reflect GluR4-subunit number at corticothalamic synapses on two populations of thalamic neurons.Proc Natl Acad Sci USA,2001,98:4172
14 Harris JD.Loudness discrimination.J Speech Hear Dis Suppl,1963,11:1
15 Harrison RV,Nagasawa A,Smith DW,et al.Reorganization of auditory cortex after neonatal high frequency cochlear hearing loss.Hear Res,1991,54:1
16 Heil P.Auditory cortical onset responses revisited.I.First-Spike Timing.J Neurophysiol,1997,77(5):2616
17 Heil P,Irvine DR.First-spike timing of audito-ry-nerve fibers and comparison with auditory cortex.J Neurophysiol,1997,78(5):2438
18 Huang CL,Lame DT,Winer JA.GABAergic organization of the cat medial geniculate body.J Comp Neurol,1999,415(3):368
19 He J,Yu YQ,Xiong Y,et al.Modulatory effect of cortical activation on the lemniscal auditory thalamus of the Guinea pig.J Neurophysiol,2002,88(2):1040
20 He J.Corticofugal modulation of the auditory thalamus.Exp Brain Res,2003,153(4):579
21 He J.Corticofugal modulation on both ON and OFF responses in the nonlemniscal auditory thalamus of the guinea pig.J Neurophysiol,2003,89:367
22 Hazama M,Kimura A,Donishi T,et al.Topography of corticothalamic projections from the auditory cortex of the rat.Neuroscience,2004,124(3):655
23 Heil P.First-Spike latency of auditory neurons revisited.Curr Opin Neurobiol,2004,14(4):461
24 Illing RB,Michler SA,Kraus KS,et al.Transcription factor modulation and expression in the rat auditory brainstem following electrical intracochlear stimulation.Exp Neurol,2002,185:226
25 Kakei S,Na J,Shinoda Y.Thalamic terminal morphology and distribution of single corticothalamic axons originating from layers 5and 6of the cat motor cortex.J Comp Neural,1994,37(2):170
26 Kim J,Morest DK,Bohne BA.Degeneration of axons in the brainstem of the chinchilla after auditory overstimulation.Hear Res,1997,103:169
27 Kimura A,Donishi T,Okamoto K,et al.Topography of projections form the primary and non-primary auditory cortical areas to the medial geniculate body and thalamic reticular mucleus in the rat.Neuroscience,1994,135(4):1325
28 Kimura A,Donishi T,Okamoto K,et al.Efferent connections of"plsterodorsal"auditory area in the rat cortex:implications for auditory spatial processing.Neuroscience,2004,128(2):399
29 Kennedy HJ,Crawford AC,Fettiplace R.Force generation by mammalian hair bundles supports a role in cochlear amplification.Nature,2005,433(24):880
30 Lee DS,Lee JS,Oh SH,et al.Cross-modal plasticity and cochlear implants.Nature,2001,409:149
31 Mainen ZF,Joerges J,Huguenard JR,et al.A model of spike initiation in neocortical pyramidal neurons.Neuron,1995,15(6):1427
32 Milbrandt JC,Holder TM,Wilson MC,et al.GAD levels and muscimol binding in rat inferior colliculus following a-coustic trauma.Hear Res,2000,147:251
33 Ojima H.Terminal morphology and distribution of corticothalamic fibers originating from layers 5and 6of cat primary auditory cortex.Cereb Cortex,1994,4(6):646
34 Plomp R.The rate of decay of auditory sensation.J Acoust Soc Am,1964,36:277
35 Penner MJ,et al.Intensity discrimination for pulsed sinusods of various frequencies.Percept Psychophy,1974,15:568
36 Penner MJ.Detection of temporal gaps in noise as a measure of the decay of auditory sensation.J Acoust Soc Am,1977,61(2):552
37 Pickles JO.An introduciton to the physiology of hearing.1988,London,San Diego:Academic Press.
38 Phillips DP.Effect of tone-pulse rise time on rate-level functions of cat auditory cortex neurons:excitatory and inhibitory processes shaping responses to tone onset.J Neurophysiol,1988,59:1524
39 Probst,R.A review of otoacoustic emissions.J.Acoust.Soc.Am,1991,89:2027
40 Phillips DP.Factors shaping the response latencies of neurons in the cat's auditory cortex.Behav Brain Res,1998,93(1-2):33
41 Riesz RR.Differential sensitivity of the ear for pure tones.Phys Rev,1928,31:867
42 Robertson D,Dexter RF.Plasticity of frequence ofganization in auditory cortex of guinea pigs with partial uniolateral deafness.J Comp Neurol,1989,282:456
43 Rouiller EM,Welker E.A comparative analysis of the morphology of corticothalamic projections in mammals.Brain Res Bull,2000,53(6):727
44 Robles L,Ruggero MA,Mechanics of the mammalian cochlea.Physiol Rev,2001,81(3):1305
45 Ran I,Miura RM,Puil E.Spermine modulates neuronal excitability and NMDA receptors in juvenile gerbil auditory thalamus.Hear Res,2003,176(1-2):65
46 Rouiller EM,Durif C.The dual pattern of corticothalamic projection of the primary auditory cortex in macaque monkey.Neurosci Lett,2004,358(1):49
47 Schacknow RR,DH Raab.Intensity discrimination of tone bursts and the form of the Weber function.Perception and Psychophysics,1973,14:449
48 Suga N,Xiao Z,Ma X,et al.Plasticity and corticofugal mndulation for hearing in adult animals.Neuron,2002,36(1):9
49 Shinkai M,Yokofujita J,Oda S,et al.Dual axonal terminations from the retrosplenial and visual association cortices in the laterodorsal thalamic mucleus of the rat.Anat Embryol,2005,210(4):317
50 Steriade M.Sleep,epilepsy and thalamic reticular inhibitory neurons.Trends Neurosci,2005,28(6):371
51 Thompson RF,Berry SD,Rinaldi PC,et al.Habituation and the orienting reflex:The dualprocess theory revisited.In:Kimmel HD,van olst EH,Ollebeke JF.(Eds.),The orienting reflex in humans.pp.21-60.Hillsdale NJ:Lawrence Erlbaum Associates
52 Vanrullen R,Guyonneau R,Thorpe SJ.Spike times make sense.Trends Neurosci,2005,28:1
53 Villa AE,Tetko IV,Dutoit P,et al.Corticofugal modulation of functional connectivity within the auditory thalamus of rat,guinea pig and cat revealed by cooling deactivation.J Neurosci Methods,1999,86(2):161
54 Winer JA,Wenstrup JJ,Larue DT.Pattens of GABAergic immunoreactivity define subdivisions of the mustached bat's medial geniculate body.J Conp Neurol,1992,319(1):172
55 Winer JA,Diehl JJ,Larue DT.Projections of auditory cortex to the medial geniculate body of the cat.J Comp Neural,2001,430:27
56 Wang J,Ding D,Salvi RJ.Functional reorganization in chinchilla inferior colliculus associated with chronic and acute cochlear damage.Hear Res,2002,168:238
57 Winer JA.Decoding the auditory corticofugal systems.Hear Res,2005,207(1-2):1
58 Xiong Y,Yu YQ,Chan YS,et al.Effects of cortical stimulation on auditory-responsive thalamic neurons in anaesthetized guinea pigs.J Physiol,2004,560:207
59 Yu YQ,Xiong Y,Chan YS,et al.Corticofugal gating of auditory information in the thalamus:an in vivo intracellular recording study.J Neurosci,2004,24:3060
60 Zurina P,Villa AE.Changes of single unit activity in the cat's auditory thalamus and cortex associated to different anesthetic conditions.Nerosic Res,1994,19(3):303
61 Zhao HB,Santos-Sacchi J.Auditory collusion and a coupled couple of outer hair cells.Nature,1999,399(6734):359
62 Zheng J.Prestin is the motor protein of cochlear outer hair cells.Nature,2000,405(6783):149
63 Zhang L,Tan AY,Schreiner CE,et al.Topography and synaptic shaping of direction selectivity in primary auditory cortex.Nature,2003,424(6945):201
64 姜泗长、顾瑞.临床听力学.北京:北京医科大学/中国协和医科大学联合出版社,1999
65 姜泗长,耳解剖学与颞骨组织病理学.北京:人民军医出版社,1999
66 梁之安.听觉感受和辨别的神经机制.上海:上海科技教育出版社,1999
67 李旭敬,吕宏光,崔万明.单侧耳聋配戴助听器后健侧耳DPOAE幅值增高.听力学及言语疾病杂志,2002,10(1):1
68 王 坚,蒋 涛,曾凡刚.听觉科学概论.北京:中国科学技术出版社,2005
免责声明:以上内容源自网络,版权归原作者所有,如有侵犯您的原创版权请告知,我们将尽快删除相关内容。